
It’s Easier Being Green
How a collaboration spanning departments and institutions brought clarity to the mystery of glassfrog transparency.
October 16, 2023 | By MICHAELA MARTINEZ
Originally published by Pratt School of Engineering
“WHAT WERE THE FROGS DOING WITH THEIR BLOOD?”
The question consumed Carlos Taboada for years. As a postdoctoral fellow at Duke University in biology and biomedical engineering, Taboada spent his days studying glassfrogs—tiny, lime-green frogs that populate portions of Central and South America and like to spend their days sleeping. A self-professed “frog guy,” he’d spent more than a decade studying different species of frogs and the biochemistry behind their coloring and fluorescence.
After earning his PhD, however, Taboada had a new goal: he wanted to understand how the aptly named glassfrogs become practically invisible.


“When glassfrogs are resting, their muscles and skin become transparent, and their bones, eyes and internal organs are all that’s visible,” said Taboada. “These frogs sleep on the undersides of large leaves, and when they’re transparent, they can perfectly match the colors of the vegetation. They barely even leave behind a silhouette.”
Taboada had the opportunity to study the clear amphibians when he joined Sönke Johnsen’s lab in 2018. Johnsen, a professor of biology at Duke, specializes in visual ecology and biophysics, and his lab investigates topics spanning bioluminescence, vision, light and camouflage in the deep sea. Johnsen had studied transparency before, but the skillset was primarily limited to marine animals.
“For a tissue to be transparent, light must pass through it without being scattered or absorbed,” explained Johnsen. “But animal tissue contains components like cells, fibers, nuclei, nerves—you know, the things that are required for life. But these things scatter light. If you’re going to be a transparent animal, you have to find a place to put all that stuff, at least temporarily.”
According to Johnsen, most transparent animals, like jellyfish, glass squids and zooplankton, can manage to become see-through because they don’t have too much of this internal machinery to camouflage. Their metabolisms are slow, their circulatory systems are simple, and they are weak and fragile, which means they don’t have to hide layers of tissues. Their environments in the ocean also help with camouflage, as any non-transparent components can blend in with the surrounding water.
“The problem with studying transparency on land is that there aren’t many animals that do it,” said Johnsen.
Taboada hadn’t been the only scientist to contact Johnsen about the mysteries behind the glassfrogs’ transparency. Around the same time Taboada joined the lab, Johnsen was approached by Jesse Delia, who was pursuing postdoctoral research at Stanford University. Delia had studied the behavior of glassfrogs for his PhD, and he’d spent countless nights observing the parental behavior of the amphibians in their native forests.
But during a research trip to Panama, Delia stumbled upon a sleeping frog during the day—an unusual occurrence due to the nocturnal schedule he’d adapted for his field work. He was surprised by the lack of blood circulating in the animal and captured a video, zooming in on the amphibian’s heart. He saw that instead of red blood circulating through the frog’s system, clear fluid was being pumped from the organ.
“As soon as I started nudging the frog to wake it up, a swell of red blood cells appeared and started circulating,” said Delia. “But when I let the frog rest and go to sleep again, the cells would disappear.”
Johnsen immediately connected the two scientists.
“It was just one of those things where the right people came together at the right time,” said Johnsen. “Carlos and Jesse generally have different personalities and research backgrounds, but they came in with a huge amount of respect for each other’s talents and abilities, and they developed a strong scientific friendship over these frogs.”
Over the next several months, Taboada and Delia set up colonies of glassfrogs—one at Stanford and one in the sub-basement of the Biological Sciences building at Duke. After several visits and countless Zoom calls, they developed a theory about how the frogs were able to become transparent.
“We could see that the frog was doing something to conceal its red blood cells, because once it removed them from circulation it could achieve transparency,” said Taboada.
This made sense. Red blood cells are especially adept at absorbing green light, which is the color of light usually reflected by plants and other vegetation. In return, these oxygen-rich cells reflect red light, making blood, and by extension the circulatory system, highly visible, especially against a bright green leaf.

The bigger question was—where were the red blood cells going? Delia and Taboada had several ideas, and they thought the most likely location was the liver, based on its size.
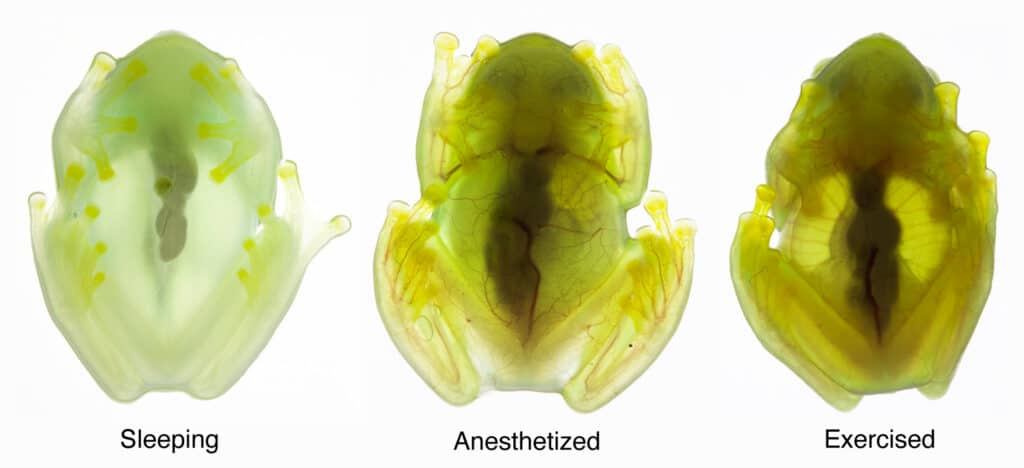
But for a see-through animal, the glassfrogs’ biology was shockingly difficult to study. “If these frogs are awake, stressed or under anesthesia, their circulatory system is full of red blood cells, and they are opaque,” explained Taboada. “The only way to study transparency is if these animals are happily asleep, which is difficult to achieve when you’re running tests on them in a research lab.”
The frog’s temperament wasn’t the only challenge. While the team was able to use traditional biological imaging tools like cameras and other optical models to quantitatively measure how the frog’s transparency increased during sleep, their technology wasn’t able to image where the frogs were storing their blood. This is because the frog’s organs are coated in a nitrogen-rich organic crystal structure. According to Taboada, frog researchers suspect that this coating helps protect the organs from damage and UV radiation from the sun. Unfortunately, it also makes them completely opaque.
“We spent a few months trying to brainstorm ways we could show where the red blood cells were going without needing to invasively test the hematocrit values of the different organs,” said Delia, who is continuing his post-doctoral research at the American Museum of Natural History in New York City. “We were really banging our heads against the wall for a solution.”
That solution came in the form of an engineer. Taboada knew about an imaging technology called photoacoustic microscopy, or PAM, from studying biliverdin, the compound that gives certain species of frogs their signature green color.
The technique involves shooting a laser into tissue. The tissue’s molecules absorb the light, heat up and expand, which causes ultrasonic waves to race back to an ultrasound sensor. These signals can be used to make detailed and colorful biomedical images of the tissue and the molecules within it.

PAM seemed like the ideal tool for tracking the red blood cells, because they wouldn’t need contrast agents or any injections that would disturb or upset the frogs. Instead, the red blood cells themselves would provide the contrast agent, because different types of cells absorb and reflect different wavelengths of light. The technique is non-invasive, quiet, sensitive, and—in a stroke of luck—available at Duke in the biomedical engineering lab of Junjie Yao.
Since joining Duke in 2016, Yao has explored methods to make PAM faster and more precise, and his lab’s state-of-the-art models can non-invasively measure the temperature of deep tissues and track deep-brain activity. Although Yao and his lab have used the technique to conduct full body scans of animal models that show blood flow, organ activity and firing neural networks, they’d never considered using it on frogs, let alone transparent ones.
“When I saw that Junjie was at Duke it seemed too perfect,” said Taboada. “I sent him an email explaining why this technique could be very useful for us and to ask if he wanted to collaborate.”
“I responded in two minutes,” said Yao, then assistant professor of biomedical engineering. “I was so excited and curious about this magic creature, and I later invited Carlos to join our photoacoustic imaging lab as a postdoc to accelerate his frog research.”
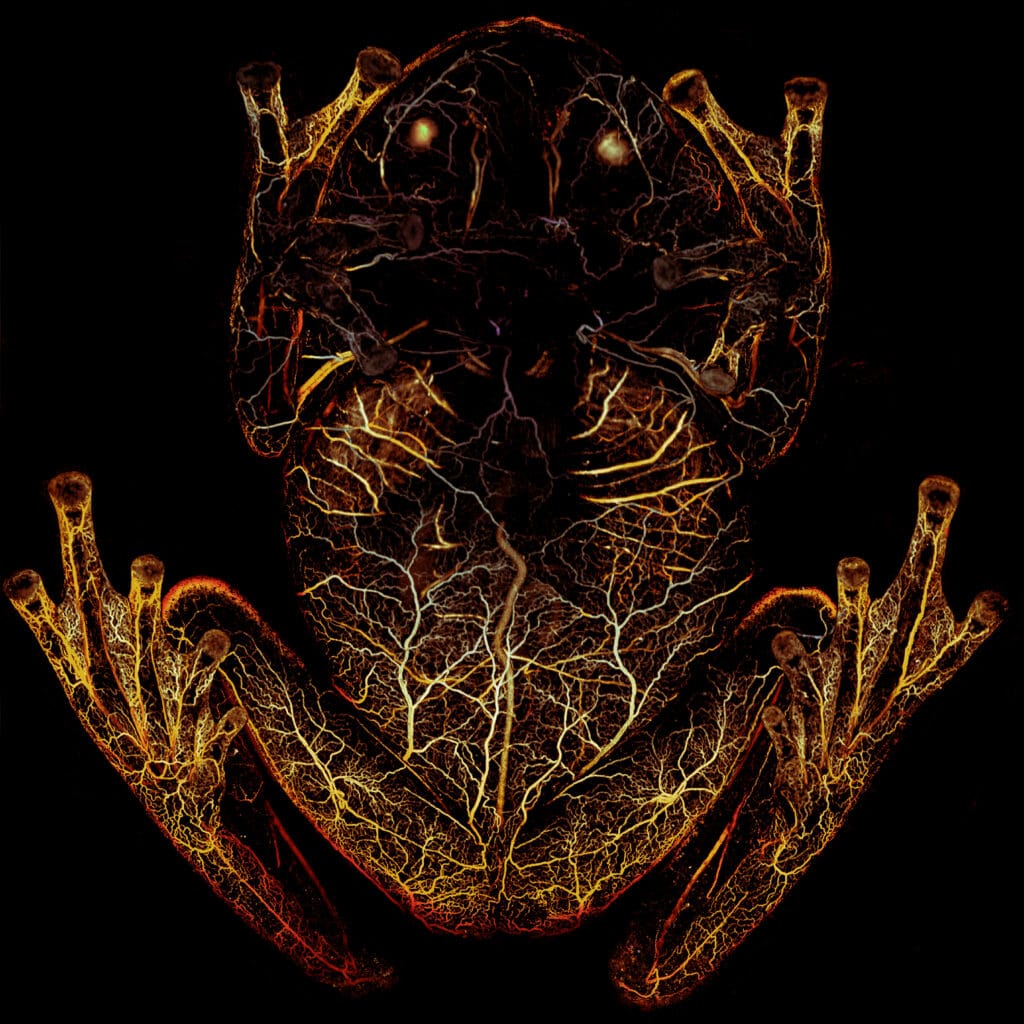
Yao adapted his platform so the team could scan the frogs by having them sleep upside down in a petri dish while Yao shined a green laser at the animal. The red blood cells in the frog’s body absorbed the green light and emitted ultrasonic waves, which were then picked up by an acoustic sensor to trace their whereabouts with high spatial resolution and high sensitivity.
“First we used high-resolution PAM, which allowed us see what happens to the individual vessels when the frog is asleep,” explained Yao. “The second technique was deep penetrating photoacoustic microscopy, and while the resolution isn’t as good, it could go deeper into the frog and image the organs.”
The results were startling.
When the frogs were asleep, they removed nearly 90 percent of their circulating blood cells, storing what is essentially their entire circulatory system in one organ—the liver.
“When the frog becomes transparent, the blood vessels fill with plasma, so the circulatory system continues to function, but with one-tenth of the blood cells that it normally uses,” said Taboada. “There’s basically no oxygen delivery to the tissue when the frogs are asleep.”
The images and videos captured with the deep-penetrating PAM showed that red blood cells flow out of the liver and circulate when the frogs were active. When the frog goes to sleep, the cells re-aggregate in the liver, turning the animal transparent in a matter of minutes.
“Biology is complicated and weird and random because of a multitude of evolutionary or species-specific reasons, where even if you study an animal and you find a reason to explain a behavior or trait, you don’t always get the full story,” said Johnsen. “But that wasn’t the case here. These results were crystal clear, and that’s so rare in biology. It was really exciting.”

The public agreed. The research appeared on the cover of Science magazine, and was featured in publications including The New York Times, The Atlantic, National Geographic, NPR, PBS and more than 400 other news sites around the globe.
While the initial question had a crystal-clear answer, the team was left with a new set of questions, the most significant of which is how the frogs manage to store 90 percent of their blood in their livers without clotting. This opens up an exciting new research avenue, one that could one day translate to address clotting and other bleeding issues in humans.
Taboada and Delia already have plans to continue to study other aspects of the frog’s biology, including how it controls its metabolism and how it prevents damage to its peripheral tissues when its oxygen-rich blood is packed away. Yao, now an associate professor of biomedical engineering, hopes to make the frog imaging work an essential part of the lab, with plans to keep developing and optimizing their large inventory of imaging tools for use on amphibians and other animal species.
“Our successful collaboration has been a great example of how multiple disciplines can jointly advance science in the most synergistic way,” said Yao. “We are extremely excited about the future directions of research we’ve opened up.”
This work, and these collaborations, will continue to extend across departments and across universities. After finishing his postdoc, Taboada accepted a faculty position at Vanderbilt University, where he’ll set up his own lab to study the biochemistry, physiology and the evolution of frogs.
“Everything I study will be related to the optics and colors of these frogs, and we’ll be able to integrate everything we’ve discovered and done through this process and learn from it,” said Taboada. “We started this work with a really simple research question about wanting to know what the frogs were doing with their blood, and now we’ve got endless opportunities.”
